Update on the Key Performance Areas
In close collaboration with Oncode Institute’s funders, a set of 5 Key Performance Areas (KPAs) has been defined to enable measurement of Oncode Institute’s performance in the current term (2023-2027).
These KPAs are organized according to Oncode Institute’s pillared strategy, together painting a coherent picture of the institute’s performance. High performance in science (KPA1) and collaboration (KPA2) provides the foundation to create impact, as represented in the valorization KPAs: the impact on patients and potential patients (Health Impact - KPA3), on the economy (KPA4), and on the affordability of new therapeutic strategies (KPA5). Each KPA is represented by at least one quantitative indicator, supported by a narrative of illustrative examples.
Scientific Excellence
Scientific excellence is the basis of Oncode Institute, an engine for breakthrough innovations in cancer treatment. With the aim of ensuring high quality scientific performance, Oncode Institute brings together ~52 leading cancer research groups in the Netherlands. Oncode Institute provides these research groups with base research funding and various...
Read more...
Collaborative Excellence
The collaborative excellence of Oncode Institute can be seen as a measure of the execution quality of its strategy to drive innovation, and contribute to affordable and sustainable healthcare through collaboration. This is based on the potential of the Oncode Institute community and the strategy to build a scientific community in...
Read more...
Health Impact
The translation of scientific discoveries into products that are implemented in clinical practice spans many years and runs through several development stages. Oncode Institute aims to achieve benefit for cancer patients and those at risk of developing cancer by accelerating the development of scientific discoveries...
Read more...
Economic Impact
The economic impact of Oncode Institute is a measure of the institute’s ability to implement a strategy which drives the translation of scientific discoveries from Oncode Investigator research activities into economic benefits. The objective for KPA4 is to establish an increase in economic impact resulting from valorization of Oncode Investigator inventions.
Read more...
Affordable and Sustainable Health Care
Oncode Institute is mindful of the need for new innovations in cancer therapy to reach patients at affordable cost. To contribute to the sustainability of the healthcare system and the affordability of new medical solutions, Oncode strives to implement feasible measures, within its capabilities, to promote affordable and sustainable healthcare. Oncode Institute will at all times adhere to its obligation to apply its best efforts to incorporate...
Read more...
KPA1: Scientific Excellence
Scientific excellence is the basis of Oncode Institute, an engine for breakthrough innovations in cancer treatment. With the aim of ensuring high quality scientific performance, Oncode Institute brings together ~52 leading cancer research groups in the Netherlands. Oncode Institute provides these research groups with base research funding and various network activities and support to perform potentially transformative research.
The objective for KPA1 is that Oncode Institute’s scientific excellence is maintained at a high level, competitive with major international research organizations in the field of biomedical and health sciences according to the Leiden Ranking1. Scientific excellence will be measured using the following indicators:
- The Mean Normalized Citation Score (MNCS): a field-normalized citation score for all Oncode Institute affiliated publications compared to the worldwide average for publications. A score of 1 means publication performance is on par with the worldwide average.
- The percentage of highly cited publications (PP(top10%)): The percentage of all Oncode Institute affiliated publications that are among the top-10 percent of the citation distribution for similar publications belonging to the same fields.
2023 Update KPA1
In 2023, Oncode Institute Base Funds continued to be highly valued by Oncode Investigators. The funds provide OIs with the flexibility to rapidly act on new ideas, initiate research lines that would otherwise be difficult to finance, accelerate new and existing research lines, and change the scope of research programs. The impact of Oncode’s base funds can be seen at multiple levels, including new research collaborations, publications, and new funding opportunities based on preliminary data generated using Base Funding.
In 2023, Oncode Investigators and their teams collectively attracted 88 competitive grants and awards with a total value of €35 million. This reflects a success rate and appreciation of scientific excellence similar to the previous year (per Oncode Investigator: 2022: 1,5 grants; €0,7M vs. 2023: 1,7 grants; €0,7M).
In 2023, all 12 junior Oncode Investigators that joined the institute in 2019 were positively evaluated by an external committee of experts for their contribution to achieving Oncode Institute’s mission, including scientific excellence. As a result, they were invited to continue their affiliation as Senior Oncode Investigators per 1 January 2024. Furthermore, the Oncode Investigator community was expanded with 10 talented new investigators, growing the research community to 62 research groups across 13 institutes per January 1, 2024.
Bibliometric analysis, using the CWTS Leiden Ranking method1, indicates that 25% of all Oncode Institute-affiliated articles published in the period 2019-2022 are among the top-10 percent of the citations distribution in their field*. In addition, an MNCS of 2,06 was achieved for this period. As indicated in the table below, the performance on both PP(top10%) and MNCS indicates that Oncode Institute’s performance on scientific excellence is in line* with the objective set for the current 5-year term.
Indicator
Reference
2018-2021 trend
Target
Status 2023 |
| 2019-2022 trend |
1. | MNCS |
2,13 |
2,0
2,06 |
2. | PP(top10%) |
Top 3 level (28%) |
|
t.b.d.* (25%) |
1 CWTS Leiden Ranking: https://www.leidenranking.com/
* Benchmarking against worldwide universities and research institutes for the reported period will be conducted once the Leiden Ranking 2024 is available (expected September 2024).
KPA2: Collaborative Excellence
The collaborative excellence of Oncode Institute can be seen as a measure of the execution quality of its strategy to drive innovation, and contribute to affordable and sustainable healthcare through collaboration. This is based on the potential of the Oncode Institute community and the strategy to build a scientific community in which interdisciplinary collaborations are natural, boundaries to collaborate are overcome, and a culture of open science is fostered. Collaborative excellence will be assessed predominantly through non-quantitative means (e.g. interviews and narratives) supported by quantitative measures (monitored indicators such as joint publications, successful collaborative grant applications, industry collaborations, network analysis, and open access publications).
The objective for KPA2 is to increase collaboration, not only between Oncode research groups, but between the Oncode community and the broader scientific community – academic, industrial, and clinical. Collaboration will be evaluated through illustrative examples and an open science indicator to assess the extent to which Oncode Institute is able to create:
- Innovative research initiatives by facilitating and promoting collaborations within the Oncode community.
- A research ecosystem in which collaborations between fundamental and clinical researchers as well as patients are stimulated and rewarded.
- A research ecosystem that drives the development of research findings into potential applications through innovative public-private partnerships.
- An open science research ecosystem in which publications, data and other types of output are shared at the earliest possible stage and made available for reuse. Open science will be measured using the following metric:
• Proportion of open access publications (PP(oa)): The percentage of Oncode Institute affiliated publications with an open access status.
2023 Update KPA2
- To facilitate and foster collaboration within the Oncode research community, between research groups and institutes and across all career levels, a total 9 events hosting almost 1500 attendees were hosted. A Brainstorm session to explore ‘outside-the-box’ collaborative projects was organized, and the institute’s Grant Officer tasked with mapping out and providing support for applications to collaborative (international) funding opportunities, participating as a project partner where applicable.
- The scope and conditions of the Clinical Proof-of-Concept program to increase incentives and drive high quality clinical collaborations were evaluated. Under the renewed program, 1 project application was approved in 2023, and additional applications were submitted. This year, there were no clinical workshops. Under the patient engagement program, 3 dedicated patient engagement meetings were organized, 3 sessions were organized at 3 Oncode Institute events, and the program manager presented 3 invited lectures. The Patient Participation program grew to 23 patients and 13 research groups.
- Proactive Industry Engagement resulted in 127 executed agreements with an industry party, together representing a total contract value of €12,7M of which industry contributions totaled €4,2M in cash and €2,5M in kind. These included 20 new collaborative public-private projects, of which 8 were under the TKI-LSH Match call. Together, the public-private research collaborations represent a total contract value of €10,6M, of which industry contributions allocated to Oncode research were €3,5M in cash and €1,1M in kind.
- In line with Oncode Institute’s Open Access Publishing Policy, the ambition is to have 100% of publications published with open access, targeting a PP(oa) of >90%. Bibliometric analysis (CWTS Leiden Ranking method1, indicates that 93% of all Oncode Institute-affiliated articles published in the period 2019-2022 are published with Open Access. As presented in the table below, this indicates that Oncode Institute’s performance on Open Science is in line with the objective set for the current 5-year term.
Indicator
Reference
2018-2021 trend
Target
Status 2023 |
| 2019-2022 trend |
|
88%
>90%
93%
1 CWTS Leiden Ranking: https://www.leidenranking.com/
KPA3: Health Impact
The translation of scientific discoveries into products that are implemented in clinical practice spans many years and runs through several development stages. Oncode Institute aims to achieve benefit for cancer patients and those at risk of developing cancer by accelerating the development of scientific discoveries resulting from Oncode Investigator fundamental research into clinical stage and onward in the development process, towards the ultimate aim of achieving clinical implementation.
The objective for KPA3 is to establish an increase in the rate of Oncode invention advancement towards the benefit of cancer patients and those at risk of developing cancer. Oncode Institute’s contribution to such health benefit will be measured using the following metric:
- The number of inventions in the active Oncode clinical portfolio, and their relative position in the development pathway towards implementation. Clinical developments can be a therapeutic, diagnostic, or recommendation for clinical guideline amendment. Clinical development is initiated upon treatment of / application to a first patient.
2023 Update KPA3
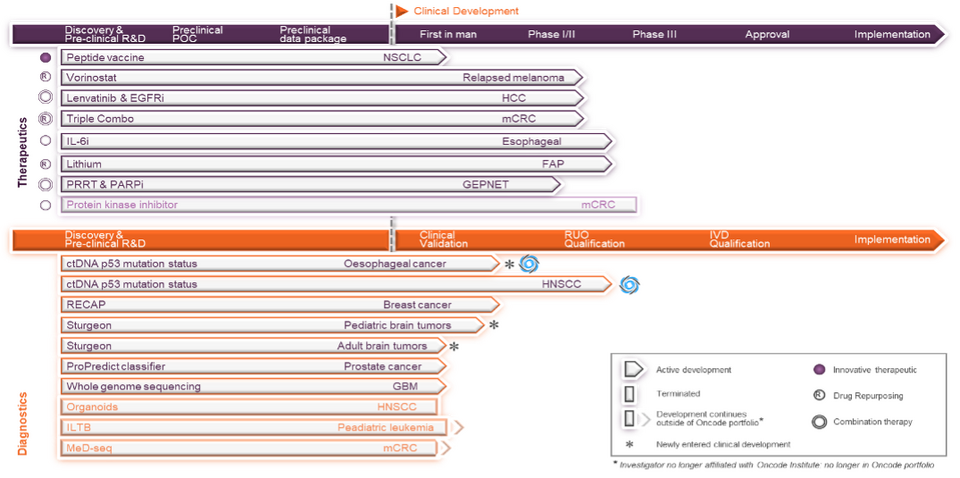
In 2023, 1 new Clinical Proof-of-Concept (CPoC) application was approved. To date, 18 CPoC projects have been approved, of which 7 are completed, 8 are actively running. In 14 projects a first patient has been included, 2 projects are approved with activities not yet started, and 1 project approval was cancelled. Starting 2023, 15 inventions were in active clinical development. Three inventions entered clinical development, all of which were readied for first-in-human clinical assessment with the support of Technology Development Funding. None were financed through the CPoC program. Clinical development of 2 inventions was discontinued due to disappointing results, 1 of which was a therapeutic and the other a diagnostic invention. The management of 2 inventions was transferred to the relevant research institute because the lead investigator’s affiliation with Oncode Institute did not continue into the current term. Consequently, these are not considered in the current active Oncode clinical portfolio. Both inventions remain in active clinical development at the respective institutes.
As summarized in the figure above and table below, at the end of 2023 the active Oncode clinical portfolio consisted of 14 inventions in active development. These represent 7 therapeutics, including 1 innovative therapeutic, 3 combination therapies, and 3 drug repurposing approaches; as well as 7 diagnostics.
Indicator
Reference
2022 end of year
Target
2027 end of year
Status 2023 |
| 2023 end of year |
Active clinical portfolio |
15 active projects
20 active projects
14 active projects
3 new, 2 terminated, 2 transferred to partner institute
KPA4: Economic Impact
The economic impact of Oncode Institute is a measure of the institute’s ability to implement a strategy which drives the translation of scientific discoveries from Oncode Investigator research activities1 into economic benefits.
The objective for KPA4 is to establish an increase in economic impact resulting from valorization of Oncode Investigator inventions. This economic impact will be measured using the following indicators:
- Leveraging of the Secured Core Funding2 invested by Oncode Institute’s funders into Oncode Institute, with private funding (which is not from a core Oncode funder) committed to either Oncode Institute or any of its spin-off companies.
- A set of internationally recognized valorization indicators developed by AUTM3 for assessment and benchmarking the quality of Oncode Institute’s valorization organization. Herein, each indicator is normalized for the total research expenditure (per 10 million euro research expenditure) within the assessed period, enabling benchmarking with international organizations of any size.
2023 Update KPA4
In 2023, in total €10,8M in cash commitment from private sources was attracted. This represents €5,4M in grants and awards from private funders (excluding any grants from Oncode Institute funder the Dutch Cancer Society), €4,1M in cash commitment from industry parties, €0,6M donations through the Major Donor program, and €0,6M investment in Oncode Institute spin-off companies. With a total Core Funding budget of €90M for this 5-year term (2023-2027), leveraging of the institute’s Core Funding with private in cash commitments thus amounts to €10,8M or 12%. As indicated in the table below, with a leveraging target of 60% in total for the current 5-year term, the leveraging performance is according to schedule.
In 2023, Oncode Investigators attracted €35M subsidy in competitive grants and awards, and with support from the Valorization Team €7,8M in cash contribution from industry parties was secured for Oncode research. The annual research budget for 2023 amounted to €49,6M, reflecting a stable relative research budget per Oncode Investigator compared to the previous year (€0,93M in 2022 versus €0,95M in 2023).
In 2023, 27 new inventions were disclosed, and 59 patent applications filed (including 15 priority applications), bringing the active patent portfolio to 59 active patent families, including 3 granted patents at year end. To support the translation of research findings into tangible health and economic benefits, €477k TechDev funding was allocated to 3 new Technology Development projects, 12 projects were successfully completed and 1 terminated, with 15 active projects at year end. During the year, 10 inventions were subject to an executed license/option agreement, with 28% of the active patent portfolio being licensed or optioned. The Oncode Oncology Bridge Fund portfolio grew with 1 spin off, in preparation of being operationalized in 2024, bringing the current portfolio to 7 spin-offs.
Reflecting on the AUTM-developed valorization indicators and Oncode Institute’s performance, North America-based technology transfer offices are well advanced and considered forerunners in valorization practice. Oncode therefore strives to perform on par with North American research institutes. As indicated in the table below, performance on new invention disclosures (i) and new spin-off companies (iv) was maintained at levels above average. Performance on new patent applications (ii) grew from below average to on par with North-American offices. Performance on new licenses and options (iii), although remaining below bench-mark, increased from 64% to 79% compared to North-American offices, indicating that Oncode Institute is on track to meet its objective for economic impact at the end of the current term.
Indicator
Reference
Target
Status 2023
1. Leveraging Core Funding |
55%4
60% (€54M) by 2027
12% (€10,8M)
2. AUTM valorization indicators
2018-2021 trend
On par with North American offices
2019-2022 trend
i. new invention disclosures
136%
maintain
160%
ii. new patent applications
81%
grow to 90%
99%
iii. new licenses and options
64%
grow to 90%
79%
iv. new spin-off companies
115%
maintain
165%
1 Discoveries from Oncode research activities are defined by (co-)inventorship on a patent application and/or a (co-)authorship on a publication.
2 Secured Core Funding is defined as the secured commitment from Oncode’s funders to the Oncode phase 2 budget as defined in the Oncode phase 2 strategy, 2022 – 2027.
3 The used set of valorization indicators developed by AUTM for benchmarking purposes, includes the following 4 indicators, each normalized per 10 million USD research expenditure in the assessed period and presented as relevant performance compared to the North American average (%): i) new invention disclosures, ii) new patent applications, ii) new licenses and options, and iv) new spin-off companies.
4 Attracted private investment in 2022 included a 17,8 million euro transaction related to the established exit of the Oncode and Hubrecht Institute spin-off company Single Cell Discoveries. This is considered an outlier value (an exit of a service-based company especially at such value is generally uncharacteristic) and is as such excluded to determine a corrected leveraging value of 0,4.
KPA5: Affordable and Sustainable Health Care
Oncode Institute is mindful of the need for new innovations in cancer therapy to reach patients at affordable cost. To contribute to the sustainability of the healthcare system and the affordability of new medical solutions, Oncode strives to implement feasible measures, within its capabilities, to promote affordable and sustainable healthcare. Oncode Institute will at all times adhere to its obligation to apply its best efforts to incorporate the NFU principles for Socially Responsible Licensing (SRL) in all license agreements it negotiates.
It is Oncode’s ambition to have the SRL principles implemented in all its licenses. In addition, Oncode’s contribution to affordable and sustainable healthcare will be assessed using the following measures:
- Allocation of at least 20% of its clinical Proof-of-Concept (CPoC) funding to drug repurposing projects.
- Mandatory health technology assessment (HTA) analysis to be conducted for all CPoC projects, where relevant.
- Mandatory use of biomarkers in CPoC projects where relevant for patient stratification or drug response monitoring.
2023 Update KPA5
In 2023, 10 inventions from the Oncode invention portfolio were licensed or optioned. This included 7 license agreements, of which 4 were for a therapeutic or diagnostic application and 3 were for a research tool. Of the licenses for therapeutic/diagnostic applications, 3 meet Socially Responsible Licensing (SRL) objectives, 2 of which specifically refer to the NFU principles of SRL. The fourth agreement reflects an amendment to a collaborative research agreement, in which a license of rights was included. Of the licenses for research tools, 1 includes specific reference to the NFU principles of SRL. Of the other 2 licenses, one grants non-exclusive rights.
During the year, under the renewed Clinical Proof-of-Concept (CPoC) program, 1 new project application to assess a new combination treatment was approved. No applications reflecting a study in the category of drug repurposing, nor patient stratification, were approved. For 1 drug repurposing CPoC project that was approved in 2021, a budget increase was approved. To date, under the Clinical program a total of €8,6M has been allocated, of which €1,8M (21%) is allocated to drug repurposing activities. In 2023, no inventions were identified that required an early Health Technology Assessment (early HTA). To date, 6 early HTAs have been initiated, all of which are now completed. The results of these early HTAs have already provided guidance for Oncode Institute’s decision process for future project development/investments.
Indicator
Reference
Target
Status 2023*
1. | CPoC funds allocated to drug repurposing |
20,8%
≥20%
0
2. | HTA for all relevant CPoC |
n/a |
100% |
n/a |
3. | Biomarker for all relevant CPoC |
n/a
100%
n/a
*In 2023 the CPoC program was re-opened, 1 first project was awarded
