Valorization
Oncode has developed an overarching valorization strategy solely focused on oncology, which is designed to accelerate the translation of new insights in cancer biology into tangible applications for patients and society. A devoted team of international experts and dedicated valorization funds are the driving forces behind Oncode’s valorization strategy. Oncode’s business developers are the first point of contact for OIs and have become an integrated part of the research groups. This has the benefit that potential breakthrough discoveries are recognized at an early stage and coupled to suitable valorization funds and tools. In 2020, the valorization team got up to full speed and is already surpassing the yearly targets set at the onset of Oncode.
183
research related agreements in 2020
17
technology development projects funded
10x
increase in TKI funding since 2018
5
spin off companies with 17 FTE employed
35%
success rate strategic funding support
3
Clinical Proof of Concept studies towards Affordable Health Care
48
new invention disclosures in 2020
17
license agreements in 2020
Impact of Technology Development fund
Through the technology development fund we aim to increase the likelihood that inventions from our labs can be further developed to increase the chances of out-licensing or creating spin-off companies. Within the programme we help our investigators to tackle scientific, technical, and business issues by financing a wide range of projects.
Read more...
Oncode Oncology
Bridge Fund
How to bridge the innovation gap? We have established the Oncode Oncology Bridge Fund to help address this question. The Bridge Fund provides pre-seed and seed capital to commercially viable enterprises originating from within our community. So far, investments were made in 5 spin-off companies employing 17 FTE.
Read more...
Oncode Strategic Funding Support
Many funding opportunities exist for oncology researchers. With our strategic funding support programme we help our Investigators to navigate this landscape. Activities range from tailored funding strategies to online course materials on grant writing, but all with the same goal: boosting Oncode research funding.
Read more...

#Valorization
Impact of Technology Development fund
Technology development fund: The Oncode Technology Development Fund (TechDev fund) was established to address scientific, technical, and business issues with the aim of increasing the likelihood that Oncode inventions can be licensed and further developed. The fund is managed by the Valorization Team and all projects are directly associated with an Oncode invention. Projects include activities such as drug target validation, high throughput screening, medicinal chemistry, toxicological studies, AD/PK studies; the formulation of drugs to support CPoC clinical trials, and health technology assessments (HTAs). With a total budget of €4M, the fund has so far funded 17 projects, of which 5 were awarded in 2020. Two projects were completed in 2020.
Carl Figdor - €149K, 2years: The work of Prof. Carl Figdor is centered around the main question of how to exploit the immune system to fight against cancer. A large focus of his research is on immunotherapy, which has emerged as a powerful and potentially curative therapy for several cancers. The manufacturing of immune cells able to stimulate the immune system is a critical step and, in its current state, a bottleneck for global implementation.
Within the lab of Prof. Figdor, a new modular, polymer-based platform technology has been developed for the activation and expansion of immune cells. While this novel technology can be used ex-vivo as the T cell activating agent in CAR-T therapy, its real novelty lies in its ability to be administered in-vivo, completely removing the need for ex-vivo manipulation. The Figdor lab has demonstrated ex-vivo and in-vivo proof-of-concept, showing that the technology can induce proliferation of T cells. A TechDev project will support the Figdor lab to establish additional proof-of-concept in multiple animal models, as well as initial safety and biodistribution studies. The aim is to create a company based on this platform technology for in-vivo and ex-vivo use, through the newly-created company’s own development programmes and interested third parties.
Mario van der Stelt – €118K, 10 months: Inearly 2020, Oncode awarded a TechDev project to Prof. Mario van der Stelt. At Leiden University, the Group of Prof. van der Stelt had identified a large number of inhibitors of a lipase that is known to promote cancer pathogenesis. The 5 most potent and selective compounds were selected for further studies. It is expected that these compounds have anti-cancer activity. Oncode coordinated the filing of a priority patent application for the inhibitors on behalf of the inventors. In the meantime, the TechDev fund provided Prof. van der Stelt with the means to select the best compounds and the best type of cancer model to enable preclinical proof-of-concept studies. By the end of 2020, all proposed experiments were completed and one compound was selected that demonstrated excellent oral bioavailability and sufficient half-life, and reached micromolar plasma concentrations. As a next step, Prof. Mario van der Stelt will perform compound testing in PDX/Xenograft models. This project illustrates how Oncode has dramatically accelerated the process from initial discovery to preclinical testing. Contingent upon successful completion of the TechDev project in 2021, Oncode will undertake further valorization activities to ensure that the compounds are progressed further towards clinics.
Mario van der Stelt (Leiden University): “The valorization team of Oncode enabled me to file a patent application for the discovery of our novel, reversible, selective and ultrapotent MAGL inhibitors (EP20161029). Furthermore, the Technology Development Fund of Oncode allowed me to profile these inhibitors in early ADME and PK studies as well as in a panel of cancer cell lines in collaboration with a biotech company.”
#Valorization
Oncode Oncology Bridge Fund
Bridge fund: The Oncode Oncology Bridge Fund, managed by Oncode B.V., is an investment fund of €7.2M that provides pre-seed and seed capital to commercially viable enterprises originating from within the Oncode community to help translate their research ideas into market-ready investment opportunities. This can include new therapeutic interventions, diagnostic screening tools and services, biomarkers, research tools or services that will benefit either cancer patients or cancer research in general. Early-stage investments are accompanied by professional intellectual property management, pre-investment technology de-risking, and, where possible, preliminary clinical validation. Additionally, the Oncology Bridge Fund prepares new enterprises for follow-on investments from private investors such as angels or venture capitalists. Oncode B.V. has committed €1.1M of investment capital in 5 spin-off companies, which currently employ 17 FTEs. In addition, 8 projects are in the ideation or validation phase. In 2020, the Oncology Bridge Fund committed €600K of investment capital in 2 spin-off companies.
Supporting aspiring entrepreneurs: In 2020, the Oncology Bridge Fund appointed 3 Entrepreneurs in Residence (EIRs) - seasoned entrepreneurs who have seen companies through from concept to successful market entry. They provide access to an extensive network of investors and business professionals. In the pre-incorporation phase, they are available for consultation with research groups to support the translation of the group’s research findings into successful business opportunities. Once the Oncology Bridge Fund has invested, the EIRs provide ongoing coaching and mentoring support to portfolio companies.
Dr. Markwin Velders brings over 25 years of research experience in the field of tumour immunology and over 18 years of management experience in biotechnology companies. He was a co-founder of T Cell Factory, which was acquired by Kite Pharma. Dr. Allard Kaptein is the Managing Director of Genase Therapeutics. Prior to that, he was co-founder of Acerta Pharma, which was acquired by AstraZeneca. Dr. Dirk Poulet brings over 25 years of experience in the diagnostics and biotechnology industries. He led Multiplicom N.V., a European diagnostics company with state-of-the-art genetic testing technology and products up until its acquisition by Agilent Technologies.
Dr. Allard Kaptein, alongside the Oncology Bridge Fund, played a pivotal role during the initiation phase of Immagene. In addition to engaging highly experienced consultants to advise Immagene on its drug discovery programmes, he mentored the company’s first CEO Maarten Ligtenberg from in-licensing the technology through to closing an investment from an investor syndicate. Dr. Kaptein continues to closely support Immagene and currently serves as an advisor and chairman of the board of directors of the company.
Oncode latest spin-off, Immagene: Originating from the lab of Prof. Daniel Peeper, Immagene was launched in September 2020 as a private biotech company, spinning out of NKI, developing next-generation precision immuno-oncology (IO) treatments. Immagene was built on findings from the Peeper lab published in Cell (2019) showing that specific components of the TNF-TRAF2 pathway can be targeted to boost immunotherapy, and a potential lead towards developing novel immunotherapeutics. Oncode drew upon its many tools to support Prof. Peeper and co-founders Dr. Ligtenberg and Dr. Blank, using its IP fund to set up two priority filings and the TechDev fund to support 4 different proof-of-concept projects that were subsequently licensed to Immagene. Furthermore, Oncode’s Entrepreneur in Residence , Dr. Allard Kaptein, provided critical advice on setting up and shaping the company. Lastly, Oncode played a pivotal role in a successful round of external funding for Immagene.
Daniel Peeper (NKI): “From the conception of this spin-off initiative, Oncode has provided support in several ways, including a dedicated business manager, financial support through the Oncode Bridge Fund, advice for non-diluted funding. Furthermore, they have brought critical expertise to the table, which turned out to be essential, even up to the very last stage prior to the launch.”
Checking back with Single Cell Discoveries: Originating from the lab of Prof. Alexander van Oudenaarden, Single Cell Discoveries (SCD) was Oncode’s first spin-off. Oncode assisted the company with incorporation, established legal agreements and provided a €110k loan for initial operating capital. Since its launch in 2018, SCD hit the ground running, expanding their team, offering new technologies, and servicing both public and private customers around the world. In 2020, 12 FTE were employed by SCD, their total revenues increased by 63% compared to 2019, and they gained more than 80 customers from 17 countries. In 2020, SCD was also part of a large European public private partnership application. The partnership brings together academic researchers (including multiple OIs), SMEs and large pharma companies.
#Valorization
Oncode Strategic funding support
Strategic funding support 101: Oncode’s Strategic Funding Support programme aims to help OIs navigate the funding landscape, thereby boosting Oncode research funding. The programme provides tailored funding strategies for OIs and spin-off companies, offers training workshops and ‘tips & tricks’ for acquiring the most frequent types of grant, and collaborates with external parties and funding bodies on training and workshops. In 2020, the programme organized a workshop entitled “get funding: Convey the impact of your scientific vision” and created online educational material in the form of a 3-part video tutorial. Funding support was provided in 43 cases, ranging from national research grants (e.g. NWO and KWF) to international grants (e.g. IMI and ERC). Of these, 35 were submitted in 2020 with a success rate of 35%. The programme has provided 5 junior and 2 senior OIs with tailored funding strategies and created funding roadmaps for 2 Oncode spin-offs. Since the programme started it has successfully secured €11,8M (see appendix II).
Coordinating large European consortia: Oncode has set itself the aim to become an internationally recognized institute for strategic initiatives in Europe and beyond. In 2020, Oncode took an important step towards achieving this goal. It successfully coordinated a large pan-European public private partnership consortium to secure a €15M Innovative Medicines Initiative grant from the European Commission.
The consortium will address therapeutic resistance, the primary cause of cancer death, which currently cannot be predicted, prevented, or treated. Therapy resistance is also a major cause of failure in the drug discovery and development process. Current experimental approaches fail to isolate and study residual disease and therapeutic resistance in clinically meaningful ways. In this project, Oncode Investigators together with their collaborators aim to develop novel single-cell sequencing techniques and advance ex-vivo and in-vivo cancer modelling approaches, which may unveil the intricate processes underlying therapeutic resistance.
Oncode took the initiative to set up and coordinate the consortium, which comprises 7 academic research teams, including 3 from Oncode (Van Oudenaarden, Clevers and Bernards), 4 SMEs and 7 major pharma companies. The set-up of this consortium not only impacts the participating Oncode research teams, it also provides a major economic impulse to 3 participating Dutch SMEs, amongst them the Oncode spin-off Single Cell Discoveries.
Tailored funding support for Junior Investigators: Navigating the funding landscape and successfully applying for new research funds can be challenging. Competition, both national and international, is fierce and the chances of success are usually slim. Oncode actively supports its research community in this complex and dynamic landscape in various ways. Specifically, Junior OIs are supported to establish their careers in today’s complex and dynamic research environment. Career stage, experience, focus area, and individual talents are all ingredients that are considered by Oncode business developers when generating tailor made, long-term funding strategies. Oncode has provided 5 Junior OIs with such support, helping them to navigate the funding landscape and tap into various funding sources at the right time. In 2020, several junior OIs put their developed strategy to use with success. A few notable examples can be found below:
Rebekka Schneider (Erasmus MC) – ERC Proof of Concept: €150K, 1.5 years – Project title: “Novel Prognostic Personalized Biomarker and Therapeutic Target in Blood Cancer Related Fibrosis”.
Job Kind (Hubrecht Institute): “The support from the valorization team in helping me with grant writing (e.g. ERC PoC together with Alexander Turkin and NWO ZonMW PSIDER with Veerle Fleskens).”
Anne Rios (PMC): “Our dedicated business developer Veerle Fleskens has helped to identify suitable funding opportunities and provided critical feedback on grant applications.”
LauraHeitman (Leiden University): “Oncode provides support in contacting and negotiating with new industrial partners. Moreover, it offers support in grant applications, which I will use for the upcoming ERC-Consolidator grant (April 2021), for example.”
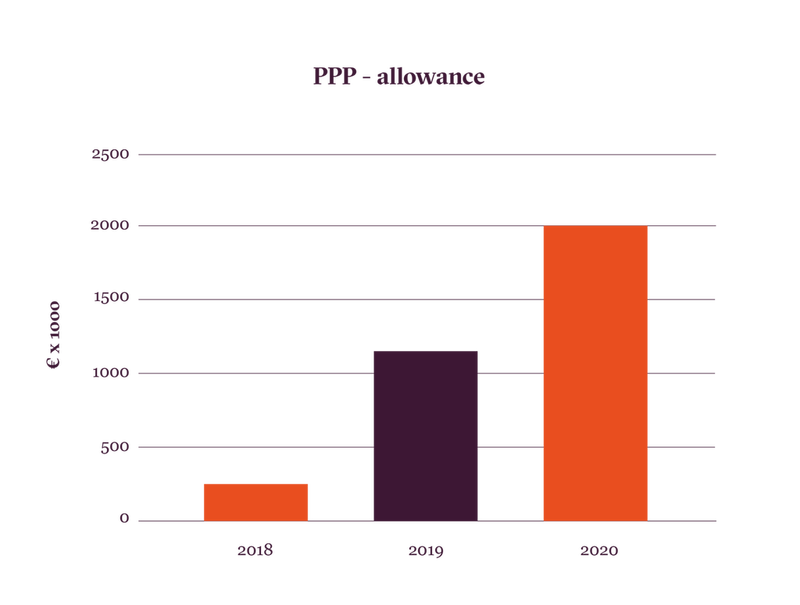
Facilitating Public Private Partnerships through PPP-allowance: In order to accelerate the translation of its research findings, Oncode has set out to proactively reach out to industry partners to build long-lasting relationships that can be utilized when opportunities for collaboration arise. Within the Netherlands, Oncode has reached out to all oncology-related SMEs to increase their awareness of Oncode and establish new relationships. In addition, Oncode has itself prominently featured during national and international meetings, and together with HollandBio organizes an annual “Biotech Wednesday” meeting, during which researchers, clinicians, and industry representatives can interact. These efforts have resulted in an increase in the number of agreements with industry (~3.5 times increase since 2018) and over 70% of OIs are now collaborating or are in discussion with industry. This is also illustrated by the dramatic increase of acquired PPP-allowance, which is provided by the Netherlands Top Sector Life Sciences & Health (LSH) to support innovative research and development realised by public-private partnerships in the Dutch LSH sector. Through the PPP-allowance, the Top Sector provides a financial instrument to help consortia that bring together research organizations, knowledge institutes, companies, and health foundations to realise their innovative ideas. Since the start of Oncode, the PPP allowance awarded to OIs has increased by 10 times (see graph).
- Alexander van Oudenaarden (Hubrecht Institute) & Single Cell Discoveries B.V.: €792k, 4 years – Project title: “A single-cell Atlas of diverse B cell clones in Chronic Lymphocytic Leukemia”
- Rene Bernards (NKI) & Oncosence B.V.: €1.016k, 2.5 years – Project title: “Exploiting senescence for the treatment of cancer”
- Hugo Snippert (UMC Utrecht) & VyCAP B.V.: €650K, 1.5 years – Project title: “Capture Tumor evolution on chip: Smart microfluidics to integrate live organoid growth with single cell genetics”
#Valorization
Affordable Health Care programme
Affordable Health Care 101: Oncode is mindful of the need for new innovations in cancer therapies to reach patients at an affordable price. While Oncode cannot solve this problem by itself, one of its goals is to contribute to the affordability of new medical solutions originating from Oncode. It therefore focusses a significant part of its strategy on this goal. Oncode’s activities in relation to Affordable Health Care (AHC) are accommodated in many different ways, including education and awareness, funds and programmes aimed at funding research that focusses on reducing the cost of care, and socially responsible licencing policies.
Investing in Clinical proof-of-concept to contribute to affordable health care: Oncode has set itself the objective to ensure that 20% of the clinical activities within its CPoC programme are focused on either personalized medicine (patient stratification) or drug repurposing. To achieve this, Oncode has funded 3 CPoC projects dedicated to AHC (pre)clinical research projects with a total budget of €~1.5M in partnership with ZonMW. In addition to the Oncode-funded CPoC projects, many OIs are involved in clinical research funded through other channels. An analysis of all the clinical research which OIs are involved in (i.e. both Oncode and non-Oncode funded research) has learned that OIs are involved in a total of 30 clinical studies which have been initiated since the start of Oncode, 50% of these studies have the potential to contribute to the affordability of healthcare. The studies either fall into the category of drug repurposing (27%) or patient stratification (23%)
The following projects are funded through the Oncode-ZonMw partnership:
Blood-borne assessments of stromal activation to guide therapy in esophageal adenocarcinoma – BASALT:A project initiated by OI Jan Paul Medema and collaborating clinician Hanneke van Laarhoven (both Amsterdam UMC). This clinical study aims to study the safety and efficacy of a new combinational therapy and validate a biomarker for esophageal adenocarcinoma. A type of cancer with a poor prognosis (a median survival of just over 3.5 years). A positive outcome from this study will provide a new treatment regimen and a much-needed stratification tool that will improve the outcome of currently available therapies.
Pulsatile high dose Sunitinib as a specific drug for (a subset of) metastatic mesenchymal CRCs:A collaboration between OI Jan Paul Medema (Amsterdam UMC) and clinician Henk Verheul (Radboud UMC). The study investigates the use of Sunitinib for metastatic mesenchymal colorectal cancer (CRC). The drug has been extensively used for metastatic renal cell carcinoma. The scientists will try to validate the laboratory observation that high doses of Sunitinib could effectively kill a wider range of cancer cells, including colorectal cell lines.
A randomized phase II study of pulsatile high-dose sunitinib versus TAS-102 in patients with metastatic colorectal carcinoma (mCRC): A follow-up study of Medema and Verheul on the above-mentioned study which demonstrated the effectiveness and safety of a pulsating high dose in patients. The investigators designed a phase II trial, for which they hypothesize that high dosed Sunitinib will result in a clinically relevant increase in the progression-free survival (PFS) period. Since Sunitinib will be off-patent at the end of 2020, the cost effectiveness of this approach will also be analyzed. If the outcome of the trial is positive, this new treatment strategy can be directly implemented in daily practice.
Providing the tools and expertise to enable drug repurposing research: Drug repurposing is an effective approach to rapidly identify novel indications for known drugs and compounds. To support researchers in bringing novel therapeutic applications to patients at affordable costs, Oncode has acquired the next-generation Drug Repurposing Library, which contains more than 6,000 drugs in various stages of clinical development (abandoned, off-patent, launched, etc.). To enable all Oncode researchers to have access to the library, Oncode has set up a Drug Repurposing programme. This programme funds drug repurposing screens and provides the technical infrastructure and expertise required to perform compound screens. In addition, copies of the library are provided to Oncode and non-Oncode researchers who have the means and expertise to perform the screens themselves. The programme was initiated in late 2019, and since then a total of 8 screening programmes from OIs have been approved by the Drug Repurposing Advisory Board, which consists of academic and industry experts in drug discovery. Two centers of expertise within the Leiden UMC and NKI perform the screens and provide guidance in setting up the assays and analyzing the results. The library has also been provided to 2 non-Oncode research projects, one of which was aimed at finding therapeutic solutions to COVID-19.
Roland Kanaar (Erasmus MC): “The Oncode Drug Repurposing Screening Programme is of great importance to extend our successful research program on hyperthermia. We discovered that the combination of a heat shock inhibitor with hyperthermia reduces heating times and temperatures required and avoids thermotolerance. Addressing these bottlenecks will increase the efficiency of hyperthermia as an anti-cancer treatment and reduces the healthcare cost per patient treated.”
Frank van Kuppenveld (Professor and Chair of Virology Division Utrecht University): “We are very pleased that we were able to access the Oncode Drug Repurposing Library and start searching for potential inhibitors of SARS-CoV-2. In addition, the smooth cooperation between the different institutes and the expertise of the Oncode screening facilities was invaluable to quickly start our search for potential virus inhibitors among already known drugs. It is nice to see that institutes like Oncode dare to cooperate with specialists from another field and therefore actively contribute to finding a solution for this health crisis."
Socially Responsible Licensing of Oncode innovations: Oncode contributed to setting up the Socially Responsible Licensing (SRL) policies and toolkit of the Netherlands Federation of University Medical Centres (NFU) and was one of the first to endorse them. The Socially Responsible Licensing (SRL) guidelines and toolkit offer Netherlands knowledge institutions a common basis for discussions with other parties about the future use of their patented knowledge. Along with the usual agreements about rights and obligations, the SRL pays explicit attention to societal objectives, such as the effective availability of products and services.
As Oncode is now gaining traction and more and more innovations are licensed to third parties, it is one of the first organizations to test the guidelines and use the tool kit in practice. In 2020, Oncode processed 17 licences, to 5 of which the SRL guidelines were applied. The SRL guidelines were not applicable to the remaining 12, because 7 of them involved in-licences or IP rights to our affiliates for their R&D use, 3 were amendments to prior license agreements, and 2 were option agreements.
